11/16/2024
Contamination of baby and infant foods
- News on contaminated products and recalls
- How to tackle the problems of safety and quality of formula
- Vulnerability of infants and young children
- Contamination of artificial milk caused by bacteria
- Contamination of baby foods caused by toxic metals and chemicals
- Breastfeeding is the best choice – even in a polluted world
- IBFAN calls for regulatory action that is long overdue
- Ten reasons for IBFAN’s Call to Action.
- Footnotes
News on contaminated products and recalls
July 2024 Preterm baby suffering from Necrotising Enterocolitis
https://www.hindustantimes.com/business/abbott-fined-500-million-by-us-court-for-allegedly-hiding-fatal-disease-risk-of-infant-formula-101722065894923.html
September 2023 AboutLawsuits.com reported: Following last year’s infant formula shortage triggered by a massive Similac cronobacter recall, which seriously sickened infants nationwide, federal health officials are investigating similar bacterial contamination problems found at three infant formula manufacturing plants responsible for the production of Enfamil, Gerber and ByHeart products.
The U.S. Food and Drug Administration (FDA) issued warning letters to Mead Johnson Nutrition (Reckitt), ByHeart Inc. and Perrigo Wisconsin, LLC on August 30, stating that routine facility inspections found evidence of cronobacter in critical hygiene zones of the infant formula manufacturers’ production plants, along with other violations. https://www.aboutlawsuits.com/enfamil-infant-formula-cronobacter-warning/
May 2023 Gerber recalling infant formula because of Cronobacter sakazakii contamination. The Perrigo Company is recalling certain lots of Gerber Good Start SootheProTM Powdered Infant Formula because of potential Cronobacter sakazakii contamination. The product was distributed in the U.S. and was manufactured at the Company’s Gateway Eau Claire, WI, manufacturing facility from Jan. 2 to 18, 2023. https://www.foodsafetynews.com/2023/05/gerber-recalling-infant-formula-because-of-cronobacter-sakazakii-contamination/
February 2023 France Probes Dairy Giant Lactalis Over Recall Failure, Negligence By Reuters Staff February 17, 2023PARIS (Reuters) – Dairy giant Lactalis is under criminal investigation in France on charges of failure to carry out product withdrawals and recalls, fraud and negligent bodily harm, the company said on Thursday. “This step marks the beginning of the judicial investigation in which Lactalis will be fully involved and in full France probes dairy giant Lactalis over recall failure, negligencetransparency”, the company said. The investigation, which will also look into the smaller company Celia Laiterie de Craon, is linked to a major scandal in 2017 involving salmonella contamination of infant formula, according to French media reports. “In the coming weeks, we will have access to all the elements in the case and we will be able to respond specifically to the totality of the points raised in this investigation,” Lactalis said. Lactalis, which is a privately held firm, is the world’s largest producer of cheese and one of the world’s largest dairy groups. Founded in 1933, the company has expanded steadily over generations. Its takeover of Italy’s Parmalat in 2011 turned it into a major global player.(Reporting by Sudip Kar-Gupta and Tassilo Hummel; Editing by Bernadette Baum) Reuters Health Information © 2023.
See also Le Monde (in English) https://www.lemonde.fr/en/france/article/2023/02/16/french-company-charged-over-baby-milk-salmonella-scandal_6016092_7.html
January 2023: Article where the FDA tries to decline its responsibility for the Abbott infant formula debacle in the US. Nothing that talks about closer monitoring of formula manufacturing, unannounced inspections, better testing protocols, etc. to avoid another contamination by Cronobacter sakazakii (formerly Enterobacter sakazakii) and/or by Salmonella.
https://www.politico.com/news/2023/01/31/fda-califf-baby-formula-reforms-00080460
October 2022: Another Abbott product recall involving the US, Canada and other American countries. https://www.similacrecall.com/us/en/2-fl-oz-recall-information.html products
September 2022: Product recall: Candia Baby Junior 4 (20-36 months), withdrawn from sale, concerns the whole of France. https://rappel.conso.gouv.fr/fiche-rappel/8202/Interne
Background: Several products (Similac, Alimentum and EleCare, as well as Similac Human Milk Fortifier) recalled by Abbott in February 2022 have caused an outbreak of serious infections in the US, with at least four children hospitalised. Three of the infections are due to Cronobacter sakazakii (formerly Enterobacter sakazakii) and one is due to Salmonella. Two deaths are under investigation by the US authorities.
May 13, 2022: Baby Formula Industry Successfully Lobbied to Weaken Bacteria Safety Testing Standards The current formula shortage is traced in part to a contamination-induced shutdown at a key manufacturing plant. https://www.foodsafetynews.com/2022/05/publishers-platform-abbott-and-public-health-officials-have-failed-in-their-duty-to-protect-the-most-vulnerable/
May 11, 2022: Abbott and public health officials have failed in their duty to protect the most vulnerable Food Safety News May22
April 28, 2022 Former employee blows whistle on baby formula production plant tied to outbreak. The document, sent to top officials at the Food and Drug Administration in October 2021, sparked outrage from U.S. Rep. Rosa DeL auro who has already demanded information from the FDA regarding the cronobacter outbreak among babies. Food Safety News April22
March 11, 2022: List of recalled products Food poison bulletin
March 1st, 2022: “The United States Food and Drug Administration, FDA, warns against using another powdered baby formula after a 2nd death”: https://www.npr.org/2022/03/01/1083696031/fda-warns-against-using-another-powdered-baby-formula-after-a-second-death?t=1646207848860 “A baby who used Abbott’s Similac PM 60/40 contracted a Cronobacter sakazakii infection and died”: https://www.insider.com/more-infant-formula-recalled-after-another-baby-dies-2022-3?utm_medium=social&utm_campaign=sf-bi-ti&utm_source=facebook.com&fbclid=IwAR3rp2WEkSfGiCVlDPr8MFpY0XvKg97kN51yl5EBsYvtNqqhW9sFVmbcjHE
February 18, 2022: Recall includes Similac Human Milk Fortifier: https://www.childrensdayton.org/the-hub/baby-formula-recall-what-you-need-know and recall in Malaysia https://www.therakyatpost.com/news/2022/02/24/kkm-recalls-2-similac-baby-formulas-due-to-bacteria-contamination/
February 17, 2022: Similac, Alimentum and EleCare powdered infant formulas of Abbott Nutrition were recalled in the USA after the first reported death as well as several reported cases of severe illness in infants, caused by contamination with the dangerous bacteria Cronobacter/Enterobacter sakazakii and Salmonella Newport. https://www.cdc.gov/cronobacter/https://efoodalert.com/2022/02/20/cronobacter-and-powdered-infant-formula/amp/ See also Frequently Asked Questions by the Centers for Disease Control (CDC) in the USA: https://www.cdc.gov/cronobacter/technical.html
Non-exhaustive list of products recalled between 2014 and 2019.
How to tackle the problems of safety and quality of formula
- See updated document on Greenfeeding 2023 – Ecofeeding and the pollution generated by artificial milks and infant formulas
- Addressing formula safety and quality issues: a review of effective measures
- IBFAN calls for regulatory action that is long overdue. These articles do not answer all the key questions about microbial contamination of powdered formulas. They do not propose mandatory measures to prevent further deaths and outbreaks of debilitating disease. Therefore IBFAN calls for regulatory action (10 questions and answers, see at the bottom of this webpage)
Vulnerability of infants and young children
Between 0 months and 3 years, infants and young children are at the most sensitive stages of the development of their brains, nervous systems and vital organs. They are therefore particularly vulnerable to bacterial infections and the effects of exposure to chemical pollutants. Unlike breast milk, artificial milk does not protect the infant from such negative effects, nor does it reduce their potential harm, firstly because artificial milk does not contain the same combination of protective and stimulating substances and therefore cannot contribute to the development of the psycho-neuro and immune system.
Contamination of artificial milk caused by bacteria
Intrinsic microbiological contamination: IBFAN/GIFA appreciates WHO’s work on the problem of intrinsic contamination of powdered foods for infants and young children. After reports increased in 2000 alerting health care professionals to mortality and morbidity caused by species of Salmonella and Enterobacter/Cronobacter, the WHA adopted two resolutions in 2005 and 2008 (WHA 58.32 and 61.20). These called on WHO to prepare guidance on safe preparation, storage and handling of powdered infant formula and WHO and FAO accordingly organized three Joint Expert meetings with FAO to discuss and produce the 2007 FAO/WHO Guidelines on safe preparation, storage and handling of powdered infant formula (PIF.)
– Inform parents
- Safe preparation, storage, and handling of powdered infant formula: guidelines https://www.who.int/publications/i/item/9789241595414
- The information for parents and caregivers on preparation of infant formula is of critical importance for emergency and relief workers and thus can also be found here: https://www.ennonline.net/infantformulaguidelines.
- Article 9.2 of the International Code on marketing of breastmilk substitutes addresses labelling and calls upon manufacturers and distributors to include instructions on labels for appropriate preparation and a warning against the hazards of inappropriate preparation.https://www.who.int/teams/nutrition-and-food-safety/food-and-nutrition-actions-in-health-systems/netcode/code-and-subsequent-resolutions
– WHO Guidelines for safe preparation
For these reasons, WHO published in 2007 the Safe Preparation, Storage and Handling of Powdered Infant Formula Guidelines. These Guidelines recommend a decontamination preparation phase to kill harmful bacteria: the water should be boiled and cooled to a temperature of not less than 70° Celsius before mixing it with the powder and let to cool down before feeding the bottle to the baby. Moreover, the remains of the bottle should be disposed of and not reused.
In response to the refusal of baby powder manufacturers to alert parents to the risks of possible contamination, IBFAN is actively working to make these Guidelines known as well as the implementation for measures taken by governments.
– Powdered infant formula is not sterile
Cases of serious infant infections have been reported for 15 years in Europe and the United States in some babies fed with 1st age and 2nd age powdered milk. In contrast to popular belief, powdered infant formula is not a sterile product and some species of bacteria of the genera Salmonella and Cronobacter can sometimes contaminate these milks, which do not undergo any final sterilization after leaving the factory. GIFA contributes to the publication of the contaminated infant food products recall list.
When the powder is mixed with warm water to prepare a bottle, even small amounts of bacteria can multiply quickly, as temperatures between 35 and 40° Celsius are favourable for their proliferation.
– Salmonella in products (Lactalis, Abbott..)
In December 2017, the Lactalis scandal exploded: 25 infants in France fell ill after consuming infant formula powder for babies contaminated with the dangerous Salmonella Agona bacteria. The call for immediate withdrawal of the products concerned affected more than 80 countries within a few weeks. The IBFAN press release (English and French) and interviews with BBC IBFAN representatives are available here.
In 2018, Infosan was to control an outbreak of Salmonellosis linked to infant formula: https://www.who.int/news/item/23-02-2018-infosan-in-action-to-control-an-outbreak-of- salmonellosis-linked-to-infant-formula.
In February 2022, Abbott recalls several products because of contamination. See above.
– Cronobacter
Cronobacter (or Cronobacter sakazakii, Enterobacter sakazakii) as well as Salmonella infections are not yet reportable diseases in all countries. This should be mandatory because formula and baby foods are distributed worldwide. The following resources about Cronobacter should be listed in relevant documents on infant feeding, with accessible weblinks:
- Enterobacter sakazakii and other microorganisms in powdered infant formula: meeting report: Microbiological Risk Assessment series 6. https://www.who.int/publications/i/item/9789241562775
- Enterobacter sakazakii) and Salmonella in powdered infant formula: meeting report: Microbiological Risk Assessment series 10 https://www.who.int/publications/i/item/9241563311
- Enterobacter sakazakii (Cronobacter spp.) in powdered follow-up formula: meeting report: Microbiological Risk Assessment series 15 https://www.who.int/publications/i/item/9789241563796
Contamination of baby foods caused by toxic metals and chemicals
Two Reports to the US Congres in 2021 alert on contamination: “Baby Foods Are Tainted with Dangerous Levels of Arsenic, Lead, Cadmium, and Mercury” and “New Disclosures Show Dangerous levels of Toxic Heavy Metals in even More Baby Foods”. Toxic chemicals are in powdered baby milk and foods – and in the water used to reconstitute them.
– Aluminium
Several scientific studies have documented the presence of not only heavy metals but also toxic metals such as aluminium in infant milks. Aluminium in infant formula (frame) / Aluminium dans les laits infantiles : https://www.asef-asso.fr/production/laluminium-ce-metal-qui-nous-empoisonne-la-synthese-de-lasef/
– Endocrine Disruptors
ED Endocrine Disruptors / PE perturbateurs endocriniens: https://www.asef-asso.fr/production/les-dialogues-de-lasef-special-perturbateurs-endocriniens/
Guide on chemical contamination and ED/Guide sur la contamination chimique et PE: https://www.asef-asso.fr/actualite/contamination-chimique-et-perturbateurs-endocriniens-lurps-et-lasef-agissent/
– Arsenic in water used to prepare powdered baby formulas and foods
The problem of toxic chemicals and heavy metals in baby foods (see above) is not limited to the USA but concerns the whole world. The risk to infant health is doubled when these commercial foods are prepared with water that may be contaminated by arsenic or other toxic metals.
Switzerland promotes the purity of its water from Alpine springs. To the Swiss, it is unthinkable that their water could be undrinkable. Yet in many Alpine regions the population is forbidden to drink the municipal tap water, no longer safe to drink because levels of arsenic in water exceed WHO standards for Tolerable Daily Intake (TDI) in drinking water. In other countries, including the USA and Pakistan, arsenic levels are far higher.
This means that infants and young children fed powdered milk and cereal commercial products may be at further risk of cumulative exposures – a double dose of arsenic in the powdered product and in the water used to reconstitute it.
– Bisphenol A
GIFA works to raise awareness of these risks associated with artificial feeding by publishing reports to the European Union and WHO, as well as articles on the IBFAN website, such as summaries of authorities’ measures to eliminate bisphenol A from rigid polycarbonate plastic baby bottles and food packaging for babies and young children.
GIFA alerts parents to the risks posed by certain chemicals in children’s food. According to reports from WHO and UNEP, several of these compounds, including bisphenol A, act as endocrine disrupters, i.e. they mimic certain human hormones such as oestrogen, helping to disrupt the hormone system and cause adverse effects on human reproduction and health.
– Elevated levels of toxic metals detected in popular drinks
A new study led by Tulane University has found that a variety of commonly consumed beverages, such as single and mixed fruit juices, plant-based milks, sodas, and teas, contain levels of toxic metals exceeding federal drinking water standards.
By measuring the concentrations of 25 toxic metals and trace elements in 60 beverages frequently found in grocery stores, the experts discovered that five of them contained levels of such potentially dangerous substances above federal drinking water standards.
Mixed-fruit juices and plant-based milks, such as almond or oat milk, contained elevated levels of toxic metals more often than other drinks. For instance, two mixed juices had levels of arsenic above the 10 microgram/liter standard, while a mixed carrot and fruit juice and an oat milk each had levels of cadmium exceeding the three parts per billion standard.
Overall, seven of the 25 elements – nickel, manganese, cadmium, strontium, selenium, and arsenic – were above the standard in some of the drinks. While lead was also detected in 93 percent of the 60 samples, most of them contained very low lead levels (below one part per billion), with the highest level (6.3 micrograms/kilogram) – detected in a lime sports drink – still not exceeding drinking water standards.
“It was surprising that there aren’t a lot of studies out there concerning toxic and essential elements in soft drinks in the United States,” said lead author Tewodros Godebo, an assistant professor of Environmental Health Sciences at Tulane. “This creates awareness that there needs to be more study.”
Although these soft drinks are most of the times consumed in smaller quantities than water – meaning that the health risks for adults are relatively low – parents should be cautious about what drinks they offer their children.
“People should avoid giving infants and young children mixed-fruit juices or plant-based milks at high volume. Arsenic, lead, and cadmium are known carcinogens and well established to cause internal organ damage and cognitive harm in children especially during early brain development,” Godebo explained. Source: Journal of Food Composition and Analysis, vol 119, June 2023 https://www.sciencedirect.com/science/article/abs/pii/S0889157523001047
Breastfeeding is the best choice – even in a polluted world
Breastmilk contains substances that help the brain to develop normally after birth. It also contains protective agents and stimulants that help the child develop a strong immune system. Breastfeeding usually serves to minimize the negative effects of exposure to chemicals during pregnancy or in the first few months of life. To make these benefits better known, as well as the risks of exposure to toxic chemicals to the body, GIFA contributed to the “Statement on Infants and Young Child Feeding and Chemical Residues,” published in 2013.
IBFAN-GIFA has published a summary with details on chemical substances in the French edition of Breastfeeding News. In 2013, GIFA also summarized the results of a major study in Norway that confirmed the benefits of breastfeeding, even in a polluted world.
Chemical residues in breastmilk
Chemical residues are found everywhere in our environment and can have a negative impact on humans and animals, especially during the prenatal period, i.e. before childbirth. The conclusion of a 2018 Swiss survey on breast milk contamination was that in all cases it is better to breastfeed your child because the benefits outweigh the risks.
In Swizterland, the analyse réalisée en 2018 par le Laboratoire fédéral d’essai des matériaux et de recherche (EMPA) reports on toxins in breast milk. Both the World Health Organisation (WHO), the EMPA chemist who carried out the analysis and a toxicologist from the Swiss Centre for Applied Human Toxicology at the University of Basel consider that the benefits of breastfeeding outweigh the risks associated with dioxin exposure: breast milk is the best food for infants and is therefore recommended without reservation. Source Promotion Allaitement Suisse (2021).
– Dioxine in breastmilk
Already in the 1990s, this topic led to studies on dioxin, in particular. The conclusion was the same: it is regrettable that breast milk is contaminated, but breastfeeding is still the best choice. https://www.letemps.ch/societe/lait-maternel-meme-contamine-reste-irremplacable (1999)
– POP (Persistent Organic Pollutants)
In 2011, Switzerland participated in a WHO/UNEP study on POPs (Persistent Organic Pollutants) in breast milk. The authors conclude: “In view of the results presented, there is no need to adapt the current recommendations on breastfeeding and feeding. Exclusive breastfeeding for 6 months is recommended for the vast majority of infants, followed by complementary breastfeeding with appropriate foods for up to two years or longer”.(page 4)
Etude OMS/PNUE sur les POP dans le lait maternel (2011)Download
– Breastfeeding to reduce pollution
Our environment is polluted and many ressources are wasted. Breastfeeding helps protect the health of mothers and babies and the health of our planet as well as our natural ressources. See our page on Green Feeding.
IBFAN calls for regulatory action that is long overdue
IBFAN’s Call to Action
IBFAN calls for mandatory warnings and information on labels and product websites that powdered formulas and human milk fortifiers are not sterile: IBFAN members’ submissions to a 2020 international survey showed that there are still very few countries where manufacturers and distributors must provide mandatory warnings on labels and information that the products are not sterile and may be contaminated by harmful bacteria.
IBFAN calls for clear and prominent information on labelling that extra care must be taken in preparation, storage and handling of powdered formulas and human milk fortifiers, and must include the lethal decontamination step.
IBFAN calls for Cronobacter/Enterobacter and Salmonella infections to become reportable as a mandatory notifiable disease in all countries. The only State in the USA that has mandatory reporting is Minnesota, and this is where the first case of Cronobacter infection was reported in the USA. If there had been no consumer reports then the public would never have known about the dangers of contaminated powdered formulas and human milk fortifiers.
Ten reasons for IBFAN’s Call to Action.
- 1. Why is IBFAN calling for action? [1]
- 2. How serious are the infections caused?
- 3. How long did the CDC know about this bacterial contamination at factory level?
- 4. Where were the recalled products exported?
- 5. What is the danger of using these recalled products?
- 6. Are these infections a rare occurrence?
- 7. Is there an increase in these infections caused by contaminated products?
- 8. Are there any steps to reduce the risk of infections caused by harmful bacteria in powdered formula?
- 9. Why is this lethal step to inactivate dangerous pathogens so critical?
- 10. Why still no warnings that powdered formulas are not sterile and “may contain bacteria that can cause serious illness in infants”? [16]
- Endnotes
1. Why is IBFAN calling for action? [1]
Because Cronobacter sakazakii and Salmonella are dangerous bacteria that thrive in lukewarm milk made with powdered formula. They can multiply and cause severe infections in babies.
This photo shows multiplication on a plate culture of reconstituted powdered formula at room temperature. In lukewarm[2] milk the bacterial growth of yellow-staining Enterobacter sakazakii, now called Cronobacter sakazakii, is exponential.
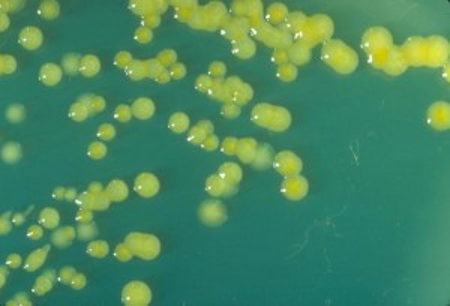
This trypticase soy agar plate culture of E. sakazakii is showing mucoid flat colonies after three days growth at 25° C. Photo credit: CDC/Dr. J. J. Farmer
2. How serious are the infections caused?
These infections can cause severe invasive infections that may be fatal.
The three products recalled by Abbott in February 2022 caused an outbreak of severe infections in the USA with at least four children hospitalised. “Three of the infections are from Cronobacter sakazakii (formerly Enterobacter sakazakii) and one is from Salmonella. One death is being investigated by US officials.”[3]
The second recall by Abbott Nutrition concerned the specialty product Similac PM 60/40: « an infant who consumed the Similac specialty product died after testing positive for Cronobacter sakazakii. It was the second reported fatal case linked to powdered baby formula since September. » See FDA Alert, March 1st 2022.
In infants less than 12 months old “Cronobacter usually causes sepsis or severe meningitis. Some infants may experience seizures. Those with meningitis may develop brain abscesses or infarcts, hydrocephalus, or other serious complications that can cause long-term neurological problems. The mortality rate for Cronobacter meningitis may be as high as 40%.” [4] See endnote 1.
3. How long did the CDC know about this bacterial contamination at factory level?
At least since 2008. The products implicated are manufactured at a production facility in Sturgis, Minnesota, USA. Abbott’s history during the 27 inspections since 2008 is examined in this article.[5] It makes chilling reading. Inspectors found positive results for Cronobacter in samples taken, as well asrepeated failures in basic hygiene, pest control, building maintenance, particle filters, temperature controls. “A review of the firm’s internal records also indicate environmental contamination with Cronobacter sakazakii and the firm’s destruction of product due to the presence of Cronobacter.”
These environmental ‘adverse inspectional observations’ resemble those found at the Lactalis factory in Craon, France, where Salmonella Agona introduced during the manufacturing process contaminated powdered formulas and baby foods.[6]
4. Where were the recalled products exported?
According to the firm (Abbott Nutrition), recalled products were distributed to the following countries: Australia, Bahrain, Barbados, Bermuda, Canada, Chile, China, Colombia, Costa Rica, Dominican Republic, Ecuador, Egypt, Guam, Guatemala, Hong Kong, India, Indonesia, Israel, Jordan, Kuwait, Lebanon, Malaysia, Mexico, New Zealand, Oman, Peru, Puerto Rico, Qatar, Saudi Arabia, Singapore, South Africa, Sudan, Taiwan, Thailand, United Arab Emirates, United Kingdom, and Vietnam ANI South.
5. What is the danger of using these recalled products?
These risks are clearly explained in “The Food and Drug investigation of Cronobacter and Salmonella complaints: powdered infant formula, 17.02.22” [7]
“Recalled powdered infant formulas have the potential to be contaminated with Cronobacter, a bacterium that can cause severe foodborne illness primarily in infants. Cronobacter infections are rare but are especially high risk for newborn infants … Cronobacter bacteria can cause severe, life-threatening infections (sepsis) or meningitis (an inflammation of the membranes that protect the brain and spine). Symptoms of sepsis and meningitis may include poor feeding, irritability, temperature changes, jaundice (yellow skin and whites of the eyes), grunting breaths, and abnormal movements. Cronobacter infection may also cause bowel damage and may spread through the blood to other parts of the body.” This invasive infection can cause bacteraemia, also known as septicaemia, in older infants.
Salmonella includes several species of bacteria causing gastrointestinal illness and fever with symptoms of diarrhoea, abdominal cramps. “More severe cases of salmonellosis may include a high fever, aches, headaches, lethargy, a rash, blood in the urine or stool, and in some cases, may become fatal.” [8]
” In the United States, the incidence of salmonellosis (from all sources) among infants (121.6 laboratory-confirmed infections per 100,000 infants) was ∼8 times greater than the incidence among other age groups”. [9]
The USFDA Alert [10] reports a law suit filed by the parents after their infant daughter « was infected with Salmonella after consuming Alimentum. Their child developed severe illness, which the lawsuit says was a “direct result” of consuming the formula. The child continues to experience gastrointestinal and bowel problems, the law firm said in a release last week. « [11]
Long-term disabilities
The risks and dangers of such infections caused by contaminated powdered formulas cannot be underestimated. Babies may suffer fatal illness, or long-term brain damage leading to life-long disabilities. In the USA, parents have taken companies to court and have received compensation to cover the costs of caring for a severely disabled child.
But no amount of money can compensate for the loss of a child or caring for a disabled child forever.
6. Are these infections a rare occurrence?
No. They are seldom reported. IBFAN calls for mandatory reporting, to make Cronobacter and Salmonella infections reportable diseases in all countries.
Baby food manufacturers repeatedly claim that cases of microbial infection caused by intrinsic contamination are rare. This is because even in the USA it is not mandatory to report Cronobacter/Enterobacter infections. However, Salmonellosis (infections caused by Salmonella) is a reportable foodborne disease.[12] In other countries there may be no or few reporting systems for food-borne illness.
The CDC admits that these infections are under-reported: “CDC typically receives reports of 2–4 infections in infants per year, although reporting is not required except in one state, Minnesota. As a result, rates of Cronobacter infection in the USA are not well understood.”[13]
Worse still, these infections remain unidentified: there are few facilities to perform tests on body fluids of sick babies and correlate these with the content of unopened powdered formula packages. Often this packaging is discarded, and the evidence disappears. Complex testing is needed to prove a causal link with the serious infections in infants and young children to powdered formula. If this is the case in the USA, then how many countries and settings lack such facilities? Even in 2002, it was noted that “Enterobacter sakazakii could be recovered from 20 out of 141 samples (14%).” [14]
7. Is there an increase in these infections caused by contaminated products?
Yes. In many countries global heating caused by climate change produces higher ambient temperatures. Warm, humid conditions increase the multiplication of any dangerous bacteria present in the reconstituted powdered formula, especially when bottles are carried around by care givers or unfinished formula bottles are kept at room temperature and consumed by babies. Increasing anti-microbial resistance makes these serious food-borne infections harder to treat. The impact of these microbial infections can include neurological damage causing life-long disabilities.
8. Are there any steps to reduce the risk of infections caused by harmful bacteria in powdered formula?
Yes. But powdered formula feeding can never be made safe. It is only possible to reduce, but not eliminate the risks. In the 2007 WHO and FAO published “New Safety Advice” with the clear and direct message: “Powdered infant formula is not sterile. It may contain bacteria that can cause serious illness in infants. By preparing and storing powdered infant formula correctly, you can reduce the risk of illness.”[15]
These Guidelines on Safe preparation, storage and handling of powdered infant formula published the same year recommend a decontamination preparation phase to kill harmful bacteria: the water should be first boiled and then cooled to a temperature of not less than 70° Celsius before mixing it with the powder and left to cool down before feeding the bottle to the baby. Bottled water should also be boiled and left to cool to no less than 70°. The remaining formula in the bottle should be disposed of and not reused.
This decontamination or lethal step is critical because both Cronobacter and Salmonella species are heat-resistant, also called thermo-tolerant. After introduction at factory level during ultra-processing, these bacteria can survive in dry state in unopened formula packages. This is called intrinsic contamination. Then, when the powdered formula is reconstituted with lukewarm water, these bacteria multiply exponentially and rapidly reach dangerous levels for infant health.
9. Why is this lethal step to inactivate dangerous pathogens so critical?
It is imperative to protect infants and young children who are at particular risk of severe infections because their immune systems are immature. Those who are not breastfed do not receive the antibodies and anti-infective agents present in breastmilk. These build the infant’s protective microbiome to boost the immune system and fight off disease.
Despite all these alerts on intrinsic contamination, manufacturers of powdered formulas and baby cereals still do not place warnings on their products. Instead, the industry opposes action at every step. Due to the opposition of the manufacturing and exporting countries the discussion in the Codex Alimentarius Committee on Food Hygiene could not reach consensus to impose mandatory warnings on labelling.
10. Why still no warnings that powdered formulas are not sterile and “may contain bacteria that can cause serious illness in infants”? [16]
It is now 20 years since the US Food and Drug Administration, FDA, issued an alert to health care professionals about contamination of powdered formulas by Enterobacter sakazakii. These harmful bacteria cause severe and potentially fatal infections in premature and newborn infants. [17]
It is over 40 years since John J. Farmer[18] documented the outbreaks of illness in babies after discovering bacteria called ‘Enterobacter cloacae’ in his dog’s feeding bowl. The name ‘cloacae’ refers to the fecal origins of these bacteria which underwent a name change to disguise their origin, becoming first Enterobacter sakazakii in 1980 and then Cronobacter sakazakii in 2007.
It is 17 years since the 2005 outbreaks of Salmonella infections in babies in France caused 148 babies to fall sick and 45% to be hospitalised. [19] The scandal of Lactalis products contaminated by Salmonella Agona hit the headlines in 2018. Potentially contaminated formulas were exported worldwide to over 83 countries. These batches were manufactured in the same facility in France where there was at least one contaminated drying tower. In the same way the batches of Abbott Similac and other powdered formulas are manufactured at their facility in Sturgis and marketed in several States and at least 37 countries.
It is now 15 years since the warning issued in the 2007 Guidelines published by WHO and FAO. Their “New Safety Advice” also issued in 2007 carries the clear and direct message: “Powdered infant formula is not sterile. It may contain bacteria that can cause serious illness in infants. By preparing and storing powdered infant formula correctly, you can reduce the risk of illness.” But how many manufacturers place these warnings on labels? How many governments mandate warnings on labelling? How many labels include the critical decontamination or lethal step? See endnote 3.
How much longer will babies fed powdered formulas have to wait? All these years and still no action. Babies cannot wait.
Endnotes
- https://corpora.tika.apache.org/base/docs/govdocs1/980/980335.html Even way back in 2002, the USFDA Letter to health professionals cites reports of severe invasive infections caused by Enterobacter sakazakii that “described neonates with sepsis, meningitis or necrotizing enterocolitis as a consequence of the infection, with case-fatality rates reported to be as high as 33%.”
- In 2018 the Lactalis scandal hit the headlines when 12 million batches of powdered formulas contaminated by Salmonella bacteria were exported to over 83 countries. IBFAN groups and contacts publicised the dangers of contaminated powdered formulas, monitored those on display or on sale, complained to health authorities and reported on any action taken: http://www.babymilkaction.org/archives/15630
- The original text of the USFDA Letter to health professionals was revised in the same year, 2002, to omit reference to using boiling water to prepare powdered formula. The baby food industry strongly opposed this step because they claimed that boiling water would destroy heat-sensitive additives to formula. Vitamin premixes and probiotics are added after the dried milk powder has been sterilised and these ingredients can introduce microbial contamination. The addition of these ingredients has been shown to be unnecessary and serves merely to justify unfounded nutritional and health claims. See https://www.foodsafetynews.com/2009/11/bacteria-in-formula-poses-risk-for-infants/
Footnotes
[1] IBFAN’s websites provide information in 3 languages: In English, French and Spanish: https://www.gifa.org/en/international-2/contaminants2/ Further articles in English: https://www.ibfan.org/contaminants-in-baby-foods/
[2] Lukewarm or tepid means 100°-110°F and 36.5°-40.5°C
[3] https://www.foodsafetynews.com/2022/02/multiple-countries-received-recalled-infant-formula-linked-to-deadly-outbreak/?fbclid=IwAR27tDozRKzt9yP3ByaTd53bqlEC3EVZrE8zO0ekuz65Yeg-RyFSkE1-Qe0
[4] https://www.cdc.gov/cronobacter/technical.html
[5] https://efoodalert.com/2022/02/20/cronobacter-and-powdered-infant-formula/amp/
[6] This recalls the 2017-2018 Lactalis scandal when the drying towers in one factory were contaminated. See endnote 2.
[7] https://www.fda.gov/food/outbreaks-foodborne-illness/fda-investigation-cronobacter-and-salmonella-complaints-powdered-infant-formula-february-2022
[8] https://www.foodsafetynews.com/2009/11/bacteria-in-formula-poses-risk-for-infants/
[9] https://academic.oup.com/cid/article/46/2/268/457688?login=false
[10] “The United States Food and Drug Administration, FDA, warns against using another powdered baby formula after a 2nd death”: https://www.npr.org/2022/03/01/1083696031/fda-warns-against-using-another-powdered-baby-formula-after-a-second-death?t=1646207848860
[11] https://www.prnewswire.com/news-releases/schlesinger-law-offices-pa-fights-for-families-in-toxic-baby-formula-class-action-lawsuit-against-abbott-laboratories-301487952.html
[12] https://www.cdc.gov/salmonella/reportspubs/surveillance.html
[13] https://www.cdc.gov/cronobacter/technical.html
[14] https://corpora.tika.apache.org/base/docs/govdocs1/980/980335.html
[15] https://www.who.int/publications/i/item/9789241595414 These 2007 Guidelines need revision to include all powdered formulas and to remove the word ‘safe’ because there is no such thing as safe formula feeding.
[16] https://www.who.int/publications/i/item/9789241595414
[17] https://corpora.tika.apache.org/base/docs/govdocs1/980/980335.html
[18] My 40-year history with Cronobacter/Enterobacter sakazakii – lessons learned, myths debunked, and recommendations
https://www.frontiersin.org/articles/10.3389/fped.2015.00084/full
[19] EuroNews – The tip of the Iceberg:https://www.euronews.com/2018/01/17/lactalis-scandal-is-a-tip-of-the-iceberg-the-international-baby-food-action-network-says
